The Importance of Sterile Packaging in the Pharmaceutical Industry
Sterile packaging is a critical aspect of the pharmaceutical industry, ensuring that medications and medical devices remain uncontaminated throughout their lifecycle—from manufacturing to patient use. This packaging not only protects products from microbial contamination but also preserves their efficacy and safety.
What is Sterile Packaging
Sterile pharmaceutical packaging is defined as a primary packaging system that holds and is in direct contact with a sterile pharmaceutical formulation throughout the shelf life of the product.It consists of a container, possibly with a closure, and is considered an integral part of the pharmaceutical product. Examples of sterile pharmaceutical packaging are vials, ampules, plastic bags, plastic bottles, etc.This blog encompasses primary packaging systems for such sterile pharmaceutical products as small-and large-volume parenterals, sterile irrigating solutions, and ophthalmic products, but not those for sterile diagnostic products and medical devices.
Key Functions of Sterile Packaging
Prevention of Contamination: Sterile packaging acts as a barrier against microorganisms, preventing contamination that could compromise the safety and effectiveness of pharmaceuticals. Contaminated products can lead to severe health issues, including infections and adverse reactions, which can be life-threatening
Maintenance of Product Integrity: Pharmaceuticals are sensitive to environmental factors such as moisture, light, and temperature. Effective sterile packaging protects against these elements, thereby preserving the chemical stability and potency of medications until they reach the end-user
Enhancing Supply Chain Security: As pharmaceuticals often travel long distances and pass through multiple handlers, sterile packaging ensures that products remain contamination-free throughout the supply chain. This is crucial for maintaining trust in healthcare delivery systems, especially during times of drug shortages
Facilitating Infection Control: In healthcare settings, sterile packaging plays a vital role in infection prevention. By ensuring that medical devices and pharmaceuticals are free from pathogens, it helps reduce the incidence of healthcare-associated infections (HAIs), which affect millions of patients annually
Considers for Choose Sterile Packaging
The primary packaging system should provide adequate protection against any ingress of foreign matter or egress of its contents, and it should possess acceptable physicochemical compatibility and long-term stability with the drug formulation within it until the drug formulation has been administered.Maintenance of a 2-to 3-year shelf-life is desirable.It is worth remembering that no container or closure is completely inert.
To make an intelligent selection of a primary packaging system that is compatible, both physically and chemically, with a sterile drug formulation, one should know about all potential instability/incompatibility problems of a packaging system with a particular drug formulation.This knowledge should derive from careful evaluation of:
- the composition of the pack-aging system;
- the treatment to which it will be subjected;
- the composition of the drug formulation.
Physicochemical interactions between sterile pharmaceutical products and their packaging components have been reported in the literature. We discuss these interactions, offering a quantitative analysis of them.Inter-actions are discussed in three categories: sorption, leaching, and permeation.In each category, discussion of the mathematical equations that are pertinent to an interaction is followed by evaluation and discussion of those critical parameters, such as temperature, that have been shown to influence the interaction. Finally, details of the interactions related to various packaging materials are presented as a handy guide for those involved in selecting a suitable primary packaging system for a formulation to achieve maximum compatibility and stability.
Primary Packaging System
The primary packaging system for a sterile pharmaceutical product consists of a container and a closure system:
Depending on composition of the materials used, containers may be classified as either glass or plastic.Their physicochemical and mechanical properties, as well as the processes involved in their manufacture, may be described as follows:
Glass containers
Glass has traditionally been considered the container material of choice for most sterile pharmaceutical products. However, it should not be assumed that glass is a totally inert material or that it is the ideal primary packaging component, either technically or commercially.
Nature and composition of glass
Glass is a noncrystalline solid and, thus, shows only short-range order to 10Å. It is also called a super cooled liquid because, under certain conditions, it can order itself and crystallize, a process known as”devitrification.”
Glass consists of a mixture of oxides.The primary glass-forming(net-work-forming)oxides are SiO₂,B₂O₃,GeO₂,P₂O₅,V₂O₅,and Al₂O₃.Among these, SiO₂is by far the major component for practically all commercial glasses.Silicone oxide is known to be the component responsible for the three-dimensional network of glass, the silicon dioxide tetrahedron. Additionally, fluxes such as CaO,Na₂O,K₂O, BaO, or Li₂O, are needed to decrease the softening temperature of glass and, there by, make it easier to process. A stabilizer, such as Al₂O₃,Sb₂O₃,PbO2, or ZnO, is also added to make the glass less prone to crystallization or devitrification and, thus, more durable.The general functions of glass formers, fluxes, and stabilizers are shown in Table I. Except for boric oxide, which can enter into the silicon dioxide tetrahedron structure, most of the inorganic oxides, such as those of sodium, potassium, calcium, magnesium, aluminum, barium, and iron, are only loosely bound in the network interstices and are, thus, relatively free to migrate.These migratory oxides can each into a drug solution that is in intimate contact with the glass container, particularly during the process of thermal sterilization.The dissolved or extracted oxides may affect solution pH, catalyze physicochemical reactions, or even enter into the reactions themselves. Additionally, some components of glass are also prone to attack by drug solutions; as a result, flakes may be dislodged into the solution.
Table I
Common Constituents of Glasses and Their Effect on Properties
| Constituent | Function | Physical & Chemical Properties |
| SiO₂ | Network former | Crystalline silica has very high melting point and liquid silica has very high viscosity. High concentration of silica in a glass confers high softening temperature, low thermal expansion, good chemical durability. |
| B₂O₃ | Network former | Will join network structure of silica glasses and reduce viscosity without producing adverse changes in thermal expansion and durability. Is a component of all heat-resisting and Chemical glasses. |
| PbO₂ | Stabilizer | Can link SiO₄ tetrahedrons. |
| AI₂O₃ | Stabilizer | Can join network in AIO₄ tetrahedron which are different in size from SiO₄. Strongly suppresses devitrification; increases process viscosity. |
| Na₂O | Network former(flux) | Markedly lowers softening temperature. Raises thermal expansion and ionic conductivity. Reduces durability. |
| K₂O | Network former(flux) | Similar to Na₂O, but the larger K+ ion is less mobile than the Na+ ion. |
| Li₂O | Network former(flux) | Similar to Na₂O, but the larger Li+ ion is less mobile than the Na+ ion. |
| CaO | Network former(flux) | inhibits mobility of alkali ions, hence increases resistivity and durability of alkali glasses. Shortens the working range. |
A true glass can be formed from the combination of SiO₂and Na₂0. A true glass is, however, soluble in water and is thus called water glass.With the addition of a stabilizer, the water solubility of true glass is greatly reduced and an insoluble soda-lime glass is formed.
Soda-lime glasses
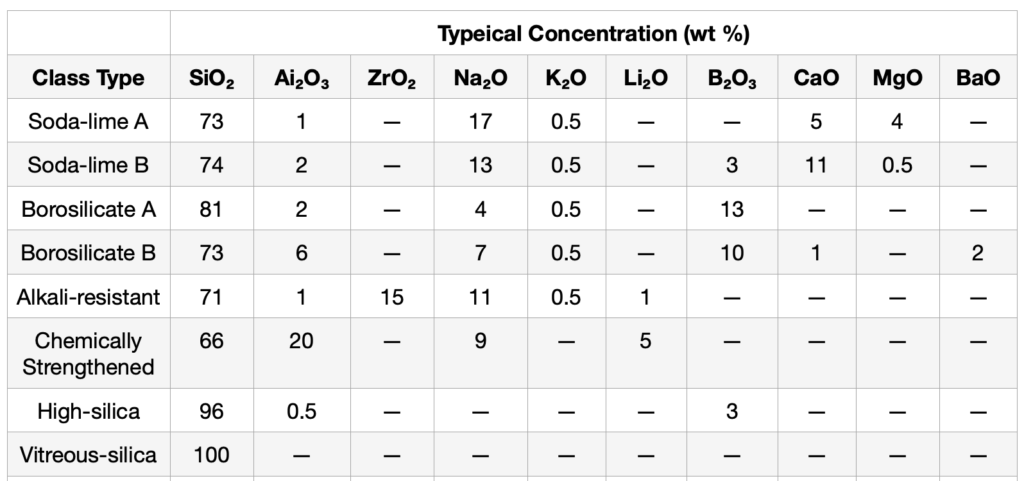
Soda-lime glasses account for approximately 90%of all commercial glasses.They are fairly resistant to chemicals, but cannot withstand sudden changes in temperature.Depending on the concentration of Na₂O,B₂O₃,CaO, and MgO in the glass network, soda-lime glasses are further classified into A and B types(Table II).
Table II
Major Chemical Constituents of Container Glasses and Glasses Used for Handling Ultrapure Solutions
The chemical resistance of soda-lime glass containers can be increased by de-alkalization of the glass surface, generally by exposing the glass toSO₂gas to remove Na₂O prior to use:
Na₂O+SO₂+½O₂→Na₂SO₄
The sodium sulfate formed remains on the surface of the glass as a fine precipitate that is water soluble and can be rinsed off easily.The de- alkalization treatment can be accelerated if SO₂ is used in the presence of H₂O.This treatment reduces the extractable alkali by a factor of 25. By means of SO₂ treatment, a soda-lime B glass(USP Type III or Type NP glass) can be upgraded to a USP Type Il glass.The chemical resistance of de-alkalized glass is comparable to that of borosilicate glass in acidic and neutral solutions, but resistance to alkaline solutions is increased only slightly by de-alkalization treatment.De-alkalized glass containers are widely used for intravenous infusion solutions.
Borosilicate Glasses
Borosilicate glasses are chemically highly resistant and are known commercially as Pyrex® and Kimax®. Typical compositions of these glasses are shown in Tables ||| and IV.
Table III
Typical Compositions (in %) of Chemically Resistant Borosilicate Glasses Manufactured by Kimble
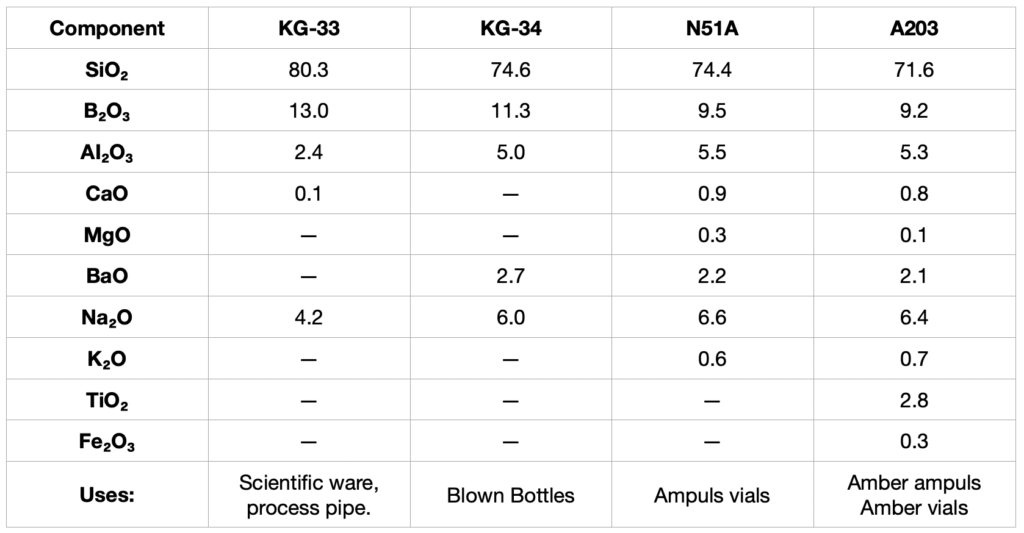
Table IV
Typical Composition (in %) of Alkali-Resistant Glasses Manufactures by Corning
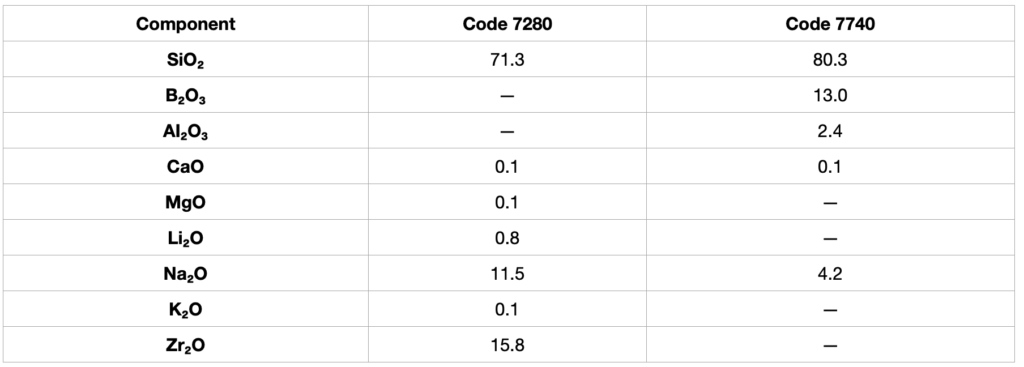
Borosilicate glasses, are also known as USP Type I glasses, can be divided into A and B subclasses ( Table II). Class A, Pyrex glass is more difficult to fabricate and has a lower thermal expansion coefficient than Class B. Class B borosilicate glass, as exemplified by the so-called neutral glass is commonly used in the manufacture of chemically resistant ampuls and vials for pharmaceuticals.
Amber Glass
Certain metals can be added to glass to produce such colors as amber, which results from an interaction between added ferric oxides and ferrous oxides and sulfur. Additional components are used to produce three types of amber glass for pharmaceutical use: reddish amber, 4% MnO₂ and 0,01% CrO₃; greenish amber, 0.1% to 1% SO₃; and brownish amber, 2% to 3% TiO₂.
Other colors can be produced by incorporating CoO for blue, NiO for gray, Cr₂O₃ for green, and CuO for bluish-green. These coloring metals in amber glass are potential sources of trace ions, particularly of iron.
Classification of glass container by USP
The United States Pharmacopeia(USP) has classified glass containers, according to their degree of chemical resistance, into four types:
Type I is made from a chemically high resistant borosilicate glass, composed principally of silicon dioxide and boric oxides. This glass has low leachability and a low thermal coefficient of expansion. In general, it is suitable for all parenteral drug products although sulfur dioxide treatment is sometimes utilized to increase its chemical resistance still more.
Type || is made from de-alkalized soda-lime glass, composed of relatively high levels of sodium oxide (13-17%) and calcium oxide (5-11%) (Table II). The existence of these two oxides makes type II glass containers chemically less resistant than Type I ( which contains 4-7% Na₂O and 1% CaO). A Type II glass container, however has a lower concentration of migratory oxides than does Type III, and its chemical resistance can be increased by sulfur dioxide treatment, under controlled conditions of temperature and humidity to de-alkalize the internal surface of the glass containers. However, this de-alkalized surface will break down if it is repeatedly exposed to heat sterilization, depylrogenation or alkaline detergents. Thus, Type II glass containers possess relatively good chemical resistance for one-time use. A Type II glass container melts at a lower temperature, can more easily be molded, and has a higher coefficient of thermal expansion than does a Type I glass container. It may be suitable for use as container for a drug solution that has been buffered to a PH below 7 or one that is not reactive with the glass.
Type III is also made from a soda-lime glass that contains relatively high levels of sodium oxide and calcium oxide, as do Type II glass containers. However, a Type III glass container has a higher concentration of migratory oxides tan does a Type II container, and it has not been subjected to de-alkalization treatment. It is usually suitable only for anhydrous liquids or of dry drug products.
Type NP is also made from a soda-lime glass and is not suitable for parenteral drug products.
Plastic Containers
Plastics fall into two general classes: thermosets and thermoplastics.
Because of their unusual versatility, thermoplastics have found wider application than thermosets especially in the medical/pharmaceutical field.
Thermoplastic polymers are gradually finding use as packaging materials for sterile preparations, such as parenteral and ophthalmic solutions. One of the principal advantages of plastic containers is that they are not as fragile as glass In addition, the flexibility of polyvinyl chloride IV bags is an advantage in the intravenous administration of large volumes of drug solution, because on air interchange is required.
Prior to the recognition of the potential of plastic materials in healthcare practice, glass was the dominant material used I the primary packaging of pharmaceutical products. The fragility and weight of glass, coupled with the broad range of properties offered by plastics, have resulted in marked increases in the use of plastics for pharmaceutical packaging during the last two decades. Today, for example, plastics are used in such sterile primary packaging systems as syringes, bottles, vials, and ampuls (Table V). many years ago, only glass would have been considered for these uses.
Table V
Sterile Plastic Devices for Parenteral Drug Administration
| Sterile Plastic Device | Plastic Material |
| Containers for blood product | Polyvinyl chloride |
| Disposable syringe | Polycarbonate Polyethylene Polypropylene |
| Irrigating solution container | Polyethylene Polyolefins Polypropylene |
| I.V. infusion fluid container | Polyvinyl chloride Polyolefins |
| Administration set | Nylon (spike) Polyvinyl chloride(tube) polymethyl methacrylate(needle adapter) Polypropylene(clamp) |
| Catheter | Teflon Polypropylene |
Polymerization
Plastic materials are prepared from monomers by polymerization. To achieve polymerization, the monomers must be bifunctional, i.e., the monomers must be capable of forming two covalent bonds. There are two classical ways in which a monomer can achieve bifunctionality: first, a monomer may contain an unsaturated C=C bond, e.g., ethylene (CH₂=CH₂); second, a monomer may possess two different organic functional groups that can react with one another, e.g., an amino acid (NH₂-CHR-COOH).
Polymerization can proceed by either of two basic processes, determined largely by the way in which the monomer has attained bifunctionality.
Polymerization by addition
Polymerization by addition ( or free radical reaction) is commonly performed with monomers that contain an unsaturated C=C bond. These monomers have the general chemical structure
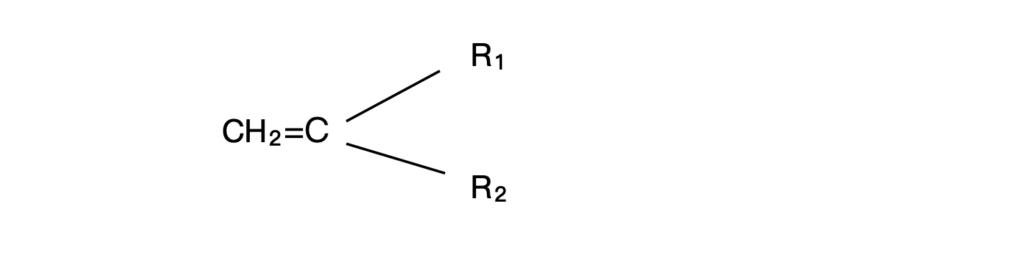
where R₁ and R₂ can be H, CH₃, phenyl, COOH, COOR, OCOCH₃, C≡N, F, CI, CONH₂, or pyrrolidone.
The polymer produced by addition polymerization may be represented as:
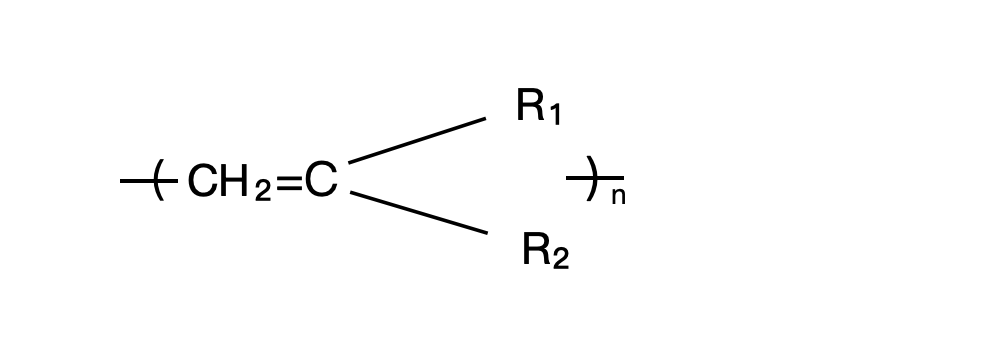
when n refers to the average number of monomer units in the polymer molecule. Depending on the chemical types of R₁ and R₂, a great variety of polymers can be produced(Table VI and Table VII). Teflon® also known as polytetrafluoroethylene, is a unique polymer produced by addition polymerization from a monomer called tetrafluoroethylene, (CF₂=CF₂)n is a unique polymer produced by addition polymerization from a monomer called tetrafluoroethylene CF₂=CF₂, in which all four hydrogen atoms in ethylene have been substituted by fluorine.
Table VI
Polymers Produced by Addition Polymerization of Vinyl-Type Monomers
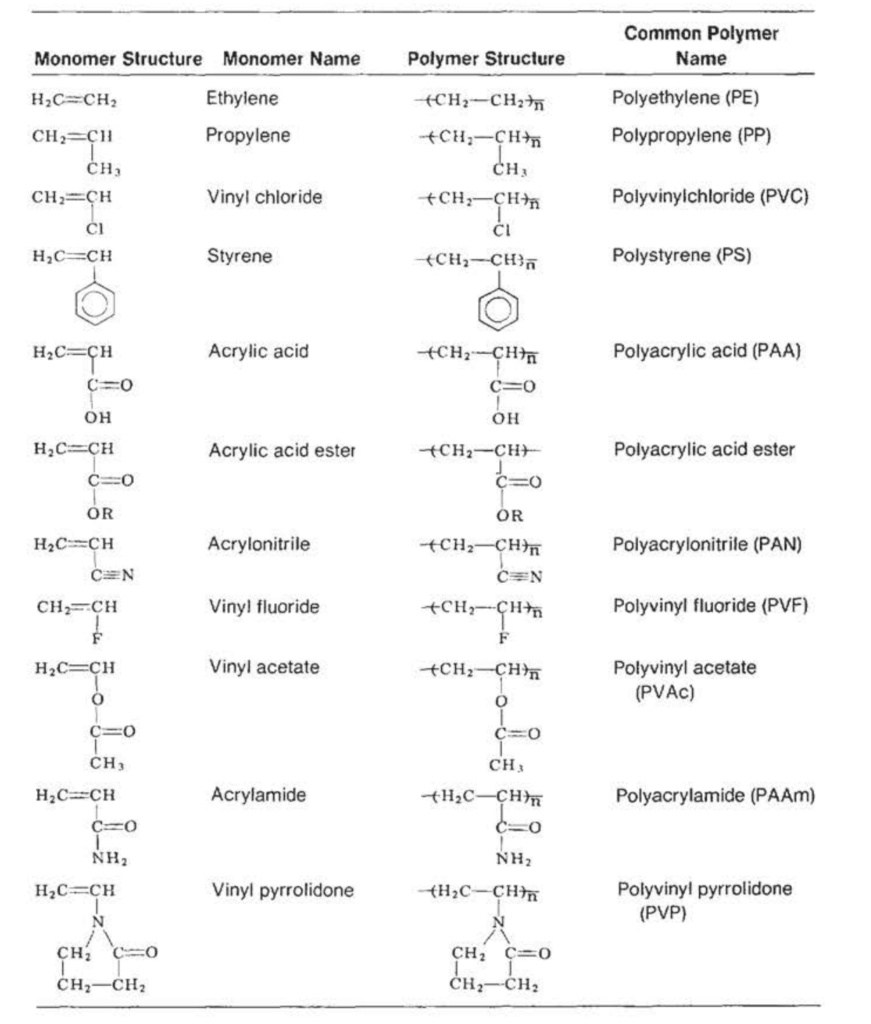
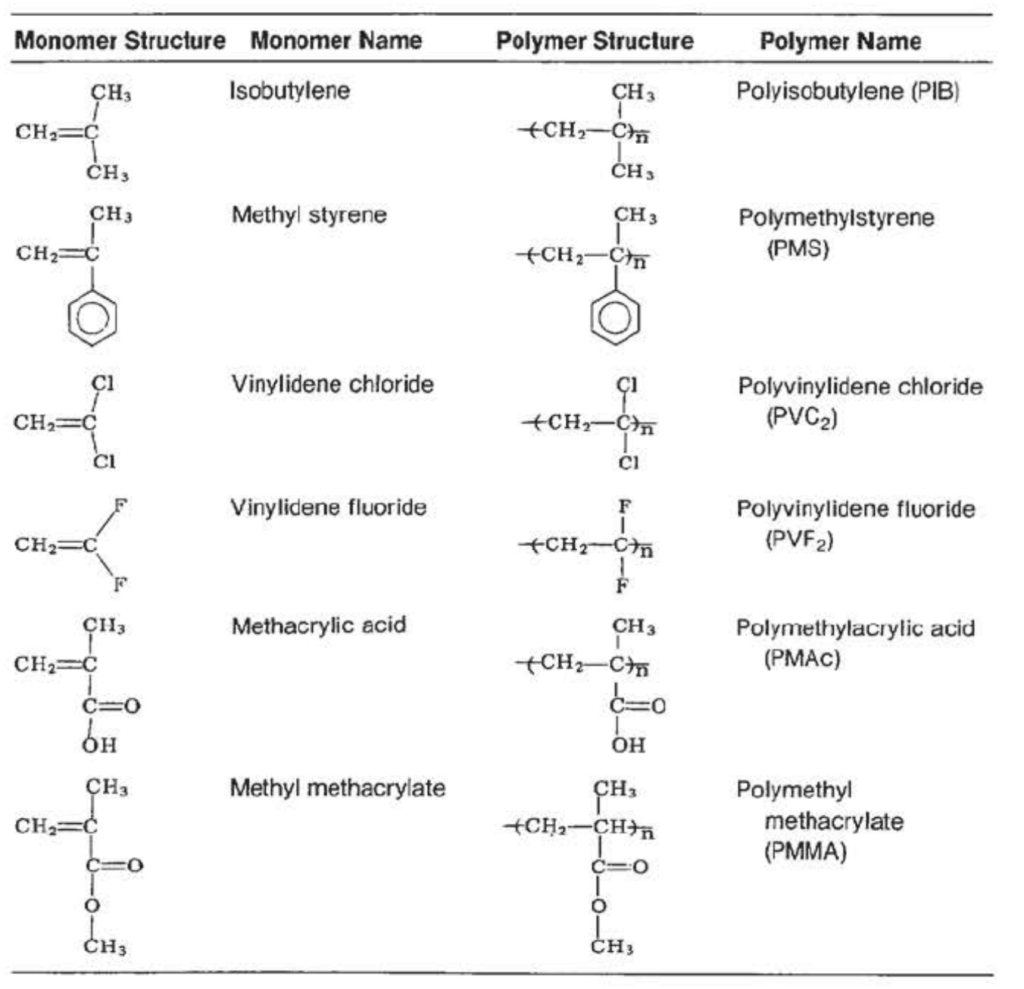
Table VII
Polymers by from Addition Polymerization of Monomers with Both R₁ and R₂ that are Not Hydrogen Atoms
Polymerization by condensation
Condensation ( or step-reaction ) polymerization commonly occurs with monomers that contain two different types of organic functional groups. It may be illustrated by the reaction of two amino acid monomers:
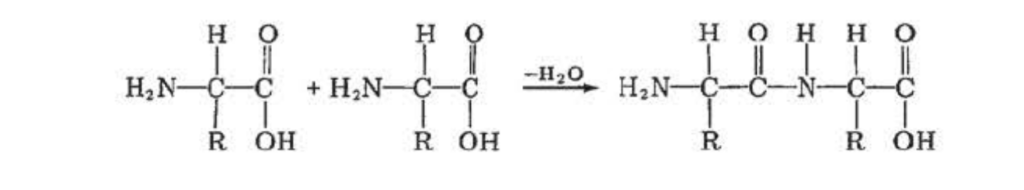
During the reaction, an amide (C—N) bond is formed and a molecule of water is condensed out. The free carboxylic (—COOH) group remaining can react with the amino group (—NH₂) of another molecule of amino acid, and the free NH₂ group can also react with the COOH group of another amino acid monomer. The polymer chain thus grows from both ends. In fact, a variety of functional group pairs can be involved to from a number of polymers via condensation polymerization ( Tables VIII and IX).
Table VIII
Some Functional Group Pairs Involved in Condensation Polymerization Reactions
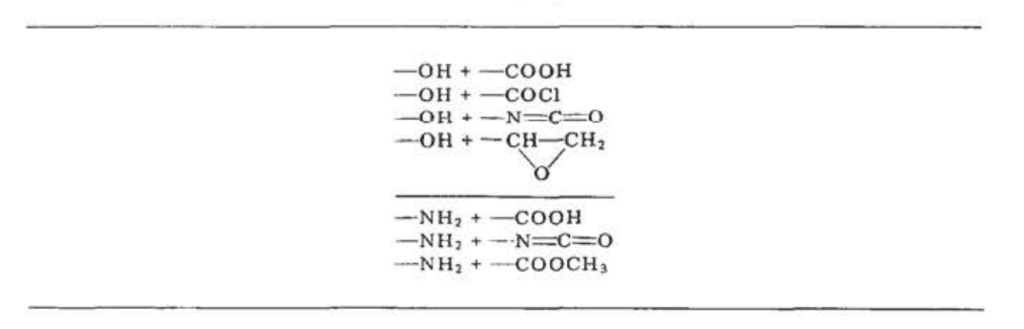
Table IX
Some Polymers Produced by Condensation Polymerization
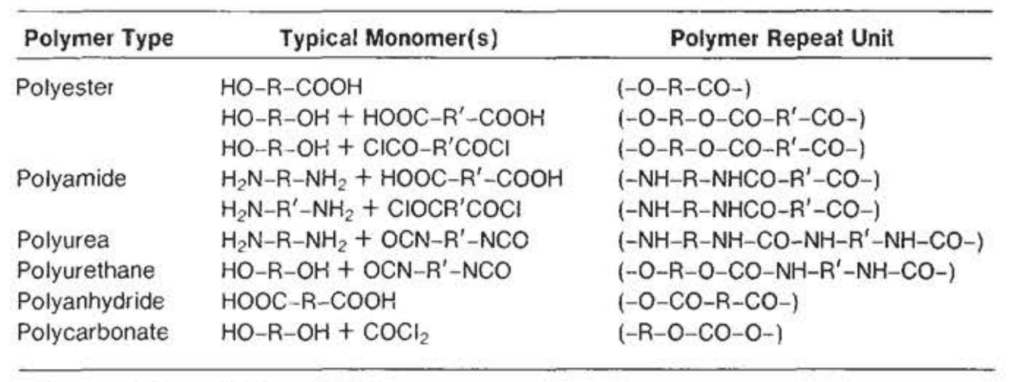
The most common examples of condensation-type polymers are polyesters, like Dacron® and Mylar®, polyamides, such as the majority of Nylons®(polypeptide is a form of polyamide), and cellulose, a condensation product of monosaccharide units.
Some monomers, such as acrylic acid ( CH₂=CH—COOH), can react through either the C=C bond or the carboxylic acid group, depending on the polymerization conditions used (Table VI).
Basically, addition polymerization occurs very rapidly after initiation and is essentially a chain reaction, whereas condensation polymerization is a much slower, stepwise reaction process. Some of the fundamental differences between these two major processed of polymerization are shown in Table X.
Table X
Comparison of Addition and Condensation Polymerization
| Addition Polymerization | Condensation Polymerization |
| Growth occurs by rapid addition of monomer to a small number of active centers | Growth occurs by coupling of any two species ( monomer or polymer) |
| Monomer concentration decreases slowly during reaction | Monomer disappears well before any high polymer is formed |
| High molecular weight polymer is present at low conversions | Polymer molecular weight increases continuously during polymerization; high polymer is present only at very high conversions. |
| Polymer back bone usually consists exclusively of carbon atoms | Polymer repeat units normally linked by oxygen and/or nitrogen atoms. |
Additives (Adjuvants)
The composition of plastic materials is quite complex. Number of plastics can be prepared for specific applications without the addition of any other ingredient to the polymer, whereas others may contain, besides the polymers, several additives to impart definite quality to the final plastic product. Specific additives are frequently used to modify the mechanical and physicochemical properties of the plastic products. The additives routinely used in thermoplastic materials may be classified as follows.
Lubricants
Lubricants are used primarily to improve the processibility of plastic materials by lowering the viscosity of melt or by preventing the polymer form sticking to the metal surfaces of the processing equipment.
Most lubricants are used in the processing of polyvinyl chloride, where they are critical to extrusion, calendaring ,injection molding, etc. Additionally, lubricants have been used in the polyolefin, styrene’s, and some thermosets. Considerable activity has recently been noted in the development of lubricants for engineering thermoplastic.
The general chemical class of lubricants used in plastic materials is alkyl acids. eg., stearic acids, and such derivatives as esters, amides, alcohol and metallic salts. For instance, commonly used lubricant in the processing of polyethylene is zine stearate. Paraffin waxes and polyethylene waxes are other popular lubricants. The quantities of lubricant used vary significantly from one plastic material to another.
Stabilizers
Stabilizers are used to retard or to prevent the deterioration of plastic materials that may result from exposure to light, heat and pressure and to improve their aging characteristics. The commonly used families of stabilizers include epoxy compounds, organotins, and mixed metals. Some of the stabilizers have some solubility in aqueous media and consequently could be extracted into a drug solution.
Plasticizers
Plasticizers are materials of low volatility that are added to plastic materials to enhance flexibility, resiliency and melt flow. They are generally high-boiling organic liquids. They act to reduce the glass transition temperature(Tg) or brittleness of the plastic to a temperature lower than that at which it will be used in an actual application. Rigid PVC, for example has a Tg of about 80°C。 Sufficient amounts of plasticizer will produce flexible PVC and decreased the Tg to below 0°C.
More than 80% of all plasticizers are used with PVC; the rest go into such plastics as cellulosic, nylon, polyolefin and styrenics. Phthalates are the most popular plasticizers. For example, 30-40% of phthalate ester is added to PVC material to produce a flexible intravenous fluid bag, such as the Viaflex and Lifecare
As is true of stabilizers, plasticizers can migrate to the surface of a plastic container and are therefore, potentically extractable into a drug solution
Antioxidants
Many plastic materials are susceptible to oxidative degradation and require antioxidants to slow down the process and to give them a longer shelf life.
Degradation of a plastic material starts with the initiation of free radicals on exposure to heat, ultraviolet radiation and mechanical shear, or in the presence of reactive impurities. Antioxidants act by intercepting the radicals or by preventing radical initiation during the shelf life of the plastic materials.
There are two types of antioxidants: Primary antioxidants act to interrupt oxidative degradation of plastics by tying up the free radicals. Such primary antioxidants as the hindered phenolics and the aromatic amines both have a reactive NH or OH group and can donate hydrogen to the free radicals. Phenolics, such as butylate hydroxytoluene(BHT) are the most popular of the primary antioxidants.BHT has a broad FDA approval and is used in polyolefin, styrene’s, vinyls and elastomers, among others.
Secondary antioxidants function by reducing the unstable hydropoweroxides formed in the plastics degradation process to inert products, thus preventing the proliferation of radicals.They are used in conjunction with primary antioxidants to provide added stability to the plastic materials.The most popular ones are thirsters and phosphates.
Antioxidants can also migrate to the surface of plastic materials and then leach out. Further, the combination of antioxidants with other additives may produce or initiate some undesirable chemical reactions with the drug solution.
Antistatic agents
Antistatic agents such as quaternary ammonium compounds are used to prevent the build-up of static charges on the surface of plastics that causes the plastic materials to cling.
Slip agents
Slip agents are added primarily to polyolefin type plastic materials, such as polyethylene and polypropylene, ignorer to reduce the coefficient of friction of the plastics. These agents impart antitank and antilock characteristics to the end products.
Dyes and pigments
Dyes and pigments impart color to plastic materials. They may leach or be extracted into a drug solution. They are used only infrequently for parenteral products.
The above-mentioned additives may vary in concentration from a few parts per million to as such as 60% of the total weight of the plastic materials.
Potential problems with plastic containers
During storage the additives described above may possibly be extracted by or leached into a drug solution that is in intimate contact with the plastic container. Therefore it is important to evaluate the physicochemical compatibility of a final drug formulation in a selected primary packaging system under various storage and time conditions to assure safety and stability of the drug product. Whenever possible evaluations should be conducted under conditions simulating those to which the product will probably be exposed. Evaluations should take into consideration not only the physical and chemical compatibility of the drug formulation with the primary packaging system, but should also include an investigation of the
mechanical properties oft he primary packaging system, e.g., the cracking and stress-cracking of a plastic container that could occur under attack by the drug product. the storage environment, or both. Prolonged exposure to ultraviolet light has been shown to promote the migration of certain additives that could. in turn, accelerate the aging characteristics of the plastic and decrease shelf life of the product.
The use of plastic material for primary packaging systems of parenteral drug products has grown very rapidly during the last two decades. With this phenomenal growth in the use of plastic containers, three potential problems have arisen:
- Loss of drug potency and the efficacy of preservation because of sorption of active drug ingredients and preservatives onto the plastic material. Such sorption has been most common in containers made of polyamides, such as nylon.
- Egress or volatile Constituents, the protective gas in the headspace, or some selective drug species through the wall of the container to the exterior. resulting in decreases in drug potency and stability; or, conversely ingress of at mospheric oxygen. water vapor, or other gases to the interior of the container. causing oxidative or hydrolytic degradation of some susceptible constituents.
- Leaching of additives or constituents from plastic containers into the drug formulation leading to a change in purity, physicochemical instability of the product. formation of particulate matter, or the causation of some adverse effect when the drug is administered.
The use of a plastic material as the primary packaging system for either a pharmaceutical or a food product demands a number of extra considerations that may not be critical to plastics employed for other purposes.
Although packaging systems for food products require considerations similar to those for pharmaceuticals, the food products usually have a relatively rapid rate of turnover and, hence, relatively short shelf-life requirements. On the other hand. pharmaceutical products require an extremely long shelf-life stability and the most critical technical and toxicological data for regulatory approval.
The pharmaceutical industry requires. in most instances. a level of safety that is more stringent than that required in the food industry. This is logical when one considers that drugs are taken by a person who is suffering from an illness: any untoward side effects may complicate the existing illness and be detrimental to health. This consideration is of particular relevance for parent era l drug products. which are to be administered directly into the systemic circulation.
Although the thermosetting resins have been widely used for making closure systems and also a few specialized containers(e.g.,menthol sticks, for almost 50 years),the use of thermoplastics for packaging pharmaceuticals did not start until the late’40s and early’50s. It is widely recognized today that plastics deservedly play a significant role in all facets of pharmaceutical packaging, provided that their advantages can be properly exploited and their potential disadvantages, in terms of physicochemical interactions with the pharmaceutical formulations, are fully evaluated and controlled.